Acetic acid is a polar protic solvent with a dielectric constant of 62 in its liquid form. Polar solvents are often found to have a high dielectric constant although other solvent scales are also used to classify solvent polarity.
Polar Vs Nonpolar Solvents Identifications And Examples Psiberg
The conductivity of a solution depends on the solvation of its ions.
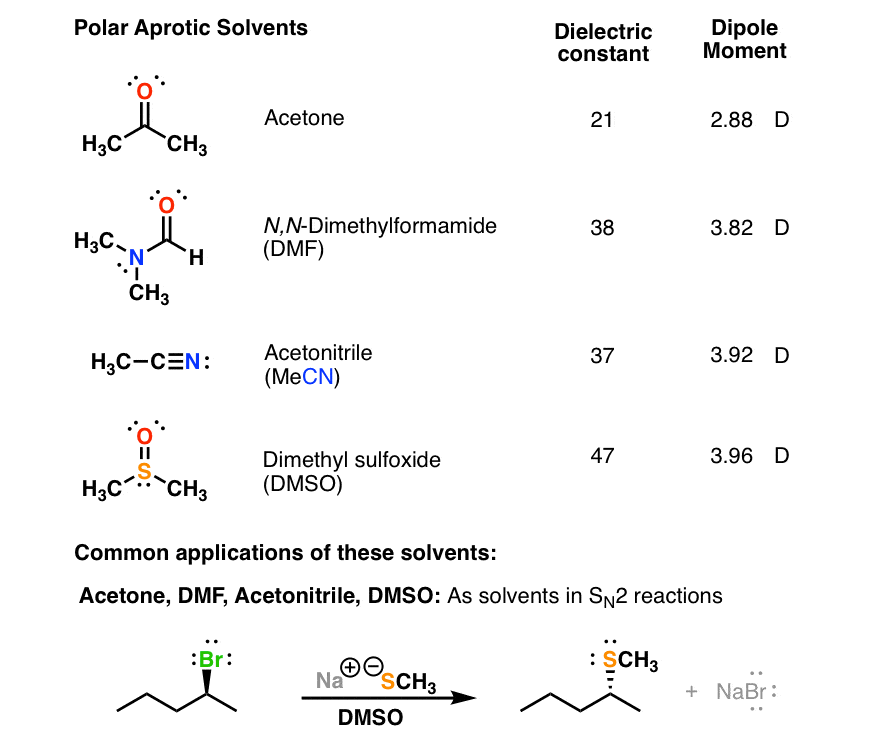
. The polarity differentiation is done based on the dielectric constant of the solvents. This is particularly true for nonpolar solvents. It is common to try three to.
Phase is nonpolar then nonpolar pigments move further up the paper. Ethers are a common example of organic phases in extractions. This is because Like dissolves like that is pigments with a chemical composition similar to the composition of the solvent will dissolve better in that solvent and therefore move further up the paper.
Also any potential hydrogen-bond acceptor will tend to shift the water signal down-field. That is solutes typically will dissolve best in solvents that have the most molecular similarities. 08 then add more nonpolar solvent or switch to an even less polar combination such as pentaneether.
For example nonpolar molecular substances like hydrocarbons are likely to be insoluble in water. The ideal solvent would have these properties. Acetic acid undergoes decomposition when heated above 440C to yield either methane and carbon dioxide or water and ethanone given by the equations.
ScCO 2 is a promising green solvent because of its negligible toxicity and high solubility window for different kind of solutes. Water-free synthesis of Ti 3 C 2 T x MXene in ammonium dihydrogen fluoride NH 4 HF 2 dissolved in polar organic solvents eg propylene. In contrast in eg.
For example nonpolar molecular substances are likely to dissolve in hexane a common nonpolar solvent. Its drawback is low boiling point of 56. For example sugar and salt polar compounds dissolve in water polar solvent but not in oil nonpolar solvent.
Water is a common example of an aqueous phase in extractions. An example of a polar solvent is water while ethanol is more nonpolar. It is also produced as a by-product in many large scale production of compounds.
The most common three types of solvents in organic chemistry are apolar polar aprotic and polar protic. DMSO the water is already strongly. Nonpolar substances are not likely to dissolve to a significant degree in polar solvents.
The nonpolar layer is referred to as the organic phase and will dissolve any nonpolar compounds. Apolar or nonpolar solvents have low dielectric constants which refers to the electric charge between the molecules being evenly distributed. It should be noted that the latter is quite temperature-dependent videinfra.
It will dissolve the compound when the solution is hot but not when the solution is cold. A solvent from the Latin solvō loosen untie solve is a substance that dissolves a solute resulting in a solutionA solvent is usually a liquid but can also be a solid a gas or a supercritical fluidWater is a solvent for polar molecules and the most common solvent used by living things. Two additional guidelines are derived from these.
All the ions and proteins in a cell are dissolved in water within the cell. Acetone CH 3 COCH 3 is an excellent solvent. For binary mixtures of acetoneisopropanol a transition from swelling to dissolution occurred near acetone volume fractions of 04505.
They found that the dissolution rate decreases with increasing solvent size indicating that dissolution rate is limited by the rate of which solvent molecules penetrate. The polar layer is referred to as the aqueous phase and it will dissolve any polar compounds. Its high diffusivity and variable density in the supercritical region replaced many conventional and toxic solvents 9It provides a green pathway for the many chemical reactions.
Nonpolar solvents cannot solvate ions and ions will. Acetic acid undergoes nearly all carboxylic acid reactions. Additionally solutes will be more soluble if the molecules in the solute are smaller than the ones in the solvent.
Shifts of the solvent residual peak2 and the water peak. Polar solvents can be used to dissolve inorganic or ionic compounds such as salts. And nonpolar gases such as ammonia ethanol and acetone at room temperature mostly because of their metallic core channels and surface functional groups that result in strong adsorption energies.
Polar solutes will dissolve better in polar solvents and nonpolar solutes will dissolve better in nonpolar solvents.
Is C3h6o Acetone Polar Or Non Polar Youtube
Polar Protic Polar Aprotic Nonpolar All About Solvents
Why Is Acetone A Good Solvent Properties Explanation Video Lesson Transcript Study Com
Polar Protic Polar Aprotic Nonpolar All About Solvents
- Contoh Laporan Projek Tahun Akhir Politeknik
- Contoh Cover Letter Untuk Apply Kerja
- Baju Kurung Malaysia Designer
- What Does Rdw Mean on a Blood Test
- undefined
- Acetone Polar or Nonpolar Solvent
- Federal Constitution Malaysia Pdf
- Electron Arrangement of Hydrogen
- Coffee 2016 Case Study Pdf
- Baju Tradisional Cina Lelaki
- Contoh Soalan Pertengahan Tahun Bahasa Melayu Tingkatan 1
